Menu
×Mica Products
Pipes & Fittings
- Column Pipe
- UPVC Pipes & Fittings
- SWR Pipes & Fittings
- Casing Pipes
- Agri Pipes & Fittings
- PVC Conduit Pipe
- Water Tanks
- Garden Pipe


Fluoroplastics are the generic term for plastic raw materials, having superb properties of heat resistance, smoothness, non-stick, chemical resistance, low friction and excellent electrical insulation. They are a critical component of modern industries with uses in transport applications and product applications like food processing, chemicals, semiconductors, liquid crystals, scientific equipment, aircraft and aerospace.
Teflon™ is the Registered trademark name for fluoroplastics made by The Chemours Company. Back in 1938, at DuPont USA, Dr. Roy Plunkett and his team discovered Teflon™.
Dr. Plunkett was researching a new refrigerant, and, as such, had stored some tetrafluoroethylene (TFE) gas in an experimental pressurized container. And, one day, when he opened the lid of the container, no gas came out; so, he cut the container in half to examine things more closely, and found that the interior walls were covered in a white powder – he had discovered PTFE.
The experiment notebook used by Dr. Plunkett then is preserved to this very day at DuPont.
During the Second World War, PTFE was used for military equipment, but in 1945 DuPont registered PTFE with the trade name Teflon™ and in 1946 production and marketing of Teflon™ goods for consumer usage began. With this the usage of Teflon™ in many industries became a possibility.
In 2015, The Chemours Company (Chemours) spun-off from DuPont and itslaunch as an independent, publicly traded corporation.
Note that in 2015 control of the Teflon™ trademark was transferred from DuPont to Chemours™. Chukoh Chemical Industries Ltd. has concluded a license agreement of Teflon™ trademark with them Chemours for a fluoroplastic adhesive tapes since July, 2017.
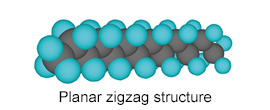
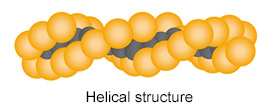
The molecular structure of PTFE provides this resin with various properties.
| Compositional formula | Steric structure | |
|---|---|---|
| PE | 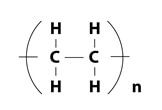 |
 |
| PTFE | 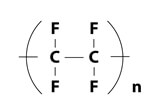 |
 |
Reason: Bonding energy of C-F bond is high
| Bond energy [kJ/mo l] | |
|---|---|
| C-H Bond (For CH₄) | 412(CH₄) |
| C-F Bond (For CF₄) | 484(CF₄) |
Reason: Intermolecular attractive force is small and surface free energy (surface tension) is low
| Type of substance | Angle of contact with water [Angle] | Adhesive energy [dyn/cm] |
|---|---|---|
| PTFE | 114 | 43.1 |
| Silicone | 490 ~ 110 | 47.8 ~ 72.7 |
| PE | 88 | 75.2 |
Reason: C-C chain is surrounded by compactly arranged fluorine atoms (F)
Reason: Polarizability of C-F bond is limited
[Remarks]
• C-F: Carbon-Fluorine bond
• CC: Carbon-Carbon bond
• Polarizability: The ease of movement of electrons when in an electric field
[Reference]
• JFIA [Fluorine resin Handbook]